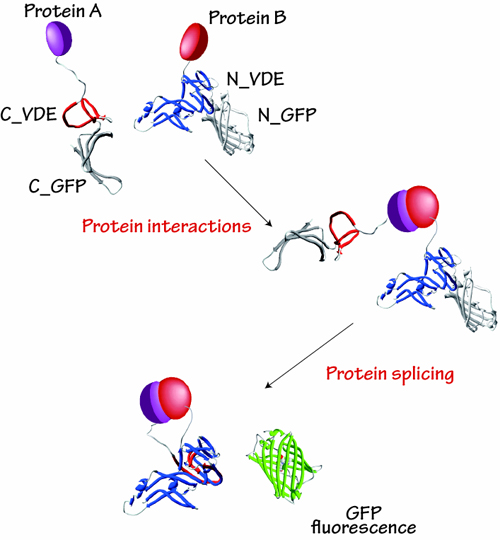
Protein-protein interactions are essential events in biological activities. Until now, biochemical methods have often been used to describe protein-protein interactions at specific times, making it difficult to track changes in protein-protein interactions that occur in vivo over time. Recently, fluorescent proteins such as GFP have been developed, but they have the disadvantage that they do not provide sufficient signals to detect protein-protein interactions due to problems such as high autofluorescence and low transparency of animal tissues, which prevent autofluorescence and light from reaching deep into the tissues.
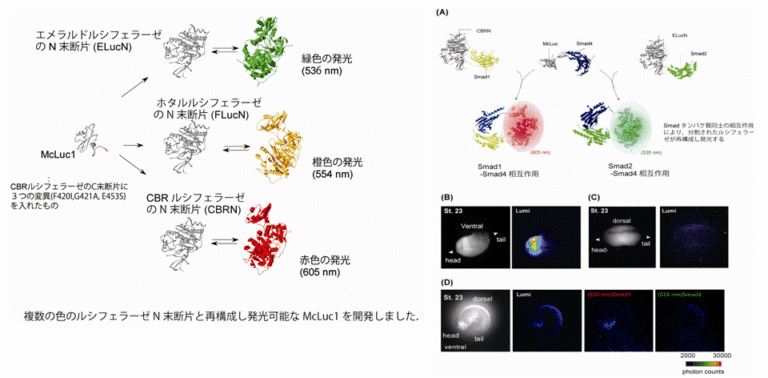
We are developing a protein-protein interaction analysis technique using bioluminescent proteins (Luciferase: Luc). When firefly-derived Luc is split in two fragments, the enzyme activity is lost. However, we have discovered that when the two fragments are brought into close proximity, the enzymatic activity of Luc is restored and the organism becomes luminescent (1). By using different species of luciferase, green, orange, and red luminescence can be separated, enabling real-time imaging of multiple types of protein-protein interactions (2). This method enables highly sensitive detection of protein-protein interactions in animals with strong and opaque autofluorescence. The developed technique enables visualization of protein localization (3) as well as intracellular signal transduction such as GPCR-βarrestin interactions (4) which will be applied to drug discovery research.
- T. Ozawa, A. Kaihara, M. Sato, K. Tachihara and Y. Umezawa, Anal. Chem., 73, 2516-2521 (2001).
- N. Hida, M. Awais, M. Takeuchi, N. Ueno, M. Tashiro, T. Singh, M. Hayashi, K. Ohmiya and T. Ozawa, PLoS ONE, 4, e5868 (2009).
- S. B. Kim, T. Ozawa, S. Watanabe, Y. Umezawa, Proc. Natl. Acad. Sci. USA., 101, 11542-11547 (2004).
- H. Takakura, M. Hattori, M. Takeuchi and T. Ozawa, ACS Chem. Biol., 7, 901-910 (2012).
