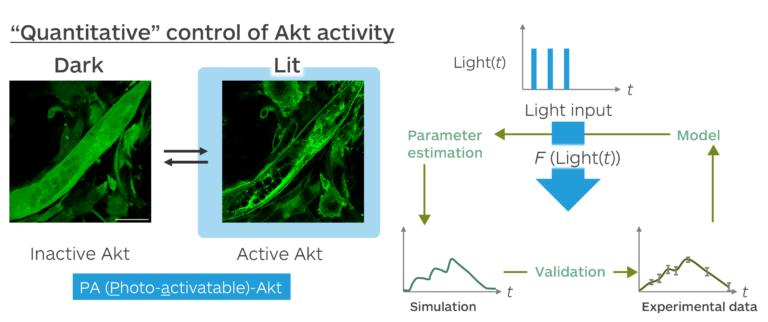
The activities of various molecules in cells rise and fall in an appropriate temporal pattern in response to the body clock and nutritional intake. For example, Akt, a protein kinase involved in intracellular signal transduction, is known to be controlled in different temporal patterns than normal in diseases such as diabetes and cancer. In order to elucidate the physiological functions of such molecular temporal patterns, it is necessary to develop a ‘quantitative method for manipulating molecular activity’ that can create various temporal patterns. To manipulate the activity of Akt, we has developed a light-responsive molecule, PA-Akt, by utilizing the photoreceptor protein CRY2 and its binding partner CIB1.
Based on the fact that Akt is activated by its translocation from the cytoplasm to the plasma membrane, PA-Akt achieves its reversible activation on a time scale of minutes by inducing light-irradiation-dependent translocation of PA-Akt to the plasma membrane. By analyzing the activation patterns of PA-Akt when irradiated with light of different intensities and patterns, we constructed a mathematical model (<Akt activity> =f[light(t)]) that can simulate the temporal patterns of Akt activation in response to arbitrary light irradiation, and succeeded in quantitatively manipulating Akt activity. Using this technique, we have shown that the gene Atrogin-1 expression in myoblasts varies depending on the temporal pattern of Akt activity.
The method of quantitative optical manipulation of molecular activity by light simulation using a mathematical model demonstrated in this study is versatile enough to be applied to the proteins other than Akt, and is expected to contribute to the development of research in general to manipulate and understand biological functions by light.
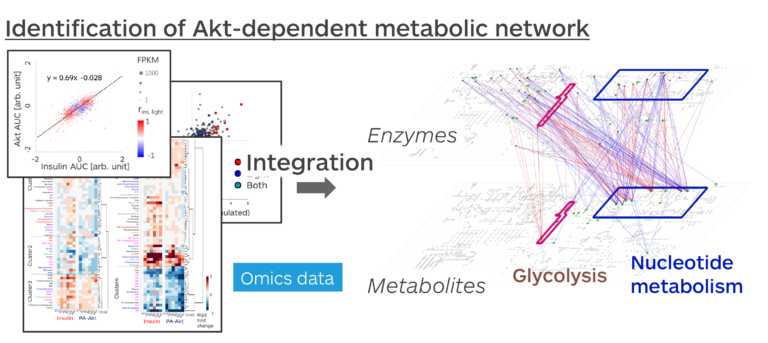
Moreover, we elucidated the function of Akt in intracellular metabolic responses using the ability of PA-Akt to induce Akt-selective activation. Metabolic responses are regulated at the cellular level by the action of insulin, a hormone that lowers blood glucose levels. Various molecules, including Akt, enzymes, and metabolites, regulate the metabolic responses activated by insulin. It is necessary to comprehensively analyze a large number of molecules to clarify which molecules contribute to the metabolic responses to what extent. In this study, we developed an analytical method that combines the selective activation of Akt by light manipulation with trans-omics analysis, which identifies molecular networks from comprehensive measurement data, to reveal the overall picture of the function of Akt in the metabolism of skeletal muscle cells.
These methods for manipulating molecular activity using mathematical model simulations based on quantitative light manipulation and for comprehensive analysis of the function of specific molecules using molecular selective-light manipulation have the potential to be applied to many molecules. They are expected to contribute to the research on “manipulating a physiological function at will to understand it,” which has been the focus of much attention in recent years.
- Y. Katsura, H. Kubota, K. Kunida, A. Kanno, S. Kuroda, T. Ozawa, Sci. Rep. 5, 14589 (2015).
- G. Kawamura, T. Kokaji, K. Kawata, Y. Sekine, Y. Suzuki, T. Soga, Y. Ueda, M. Endo, S. Kuroda, T. Ozawa. Optogenetic decoding of Akt2-regulated metabolic signaling pathways in skeletal muscle cells using transomics analysis. Sci. Signal. 16, eabn0782 (2023).
