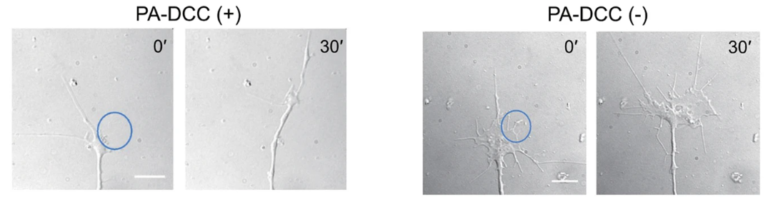
Living cells respond to changes in their surrounding environment by transmitting external chemical and physical signals into the cell via receptor proteins on the plasma membrane. Therefore, to elucidate the biological roles of these dynamic signal transductions, it is necessary to develop techniques to modulate signal transduction at arbitrary timing and locations. We are developing a tool for regulating cellular signals through receptors by light. Of the receptors responsible for chemical signal transduction, many of them are activated by dimer or oligomer formation through the binding of ligand molecules. For example, DCC, a ligand for axon guidance, is known to be activated by the oligomer formation of DCC through the binding of netrin-1. Based on the knowledge, we developed a light-responsive molecule, PA-DCC, by fusing CRY2 to the C-terminal side of DCC, which forms a oligomer upon irradiation with blue light. By introducing this molecule into neurons, we succeeded in inducing the direction of neuronal axon outgrowth in C. elegans by external light.
Tension transmission between cells is a typical physical signal transduction between cells. It is known that the plasma membrane protein E-cadherin forms a complex with E-cadherin expressed on neighboring cells at the cell-cell contact surface and transmits the contractile force generated by the actomyosin complex interacting within the cell as tension to neighboring cells. We developed a photocleavable molecule, PC-Cadherin, in which the intracellular domain of E-cadherin is cleaved by light irradiation of PhoCl. E-cadherin intracellular domain is cleaved by light irradiation. As a result, the E-cadherin complex-mediated tension transduction can be inhibited in a light-dependent manner.
It is known that the amount of receptors on the plasma membrane is strictly regulated in vivo. For example, it is known that GPCRs interact with β-arrestin on the cytoplasmic side after binding to the ligand molecule and are taken up into the cell through the endocytosis pathway. We have developed a tool to induce light-dependent interaction between β2 adrenergic receptor (ADRB2) and β-arrestin by utilizing a pair of proteins, CRY2/CIB1, which interact in a blue light-radiation-dependent manner. This tool revealed that the interaction with β-arrestin is sufficient for ADRB2 endocytosis and that the post-endocytosis pathway changes depending on the length of the interaction.
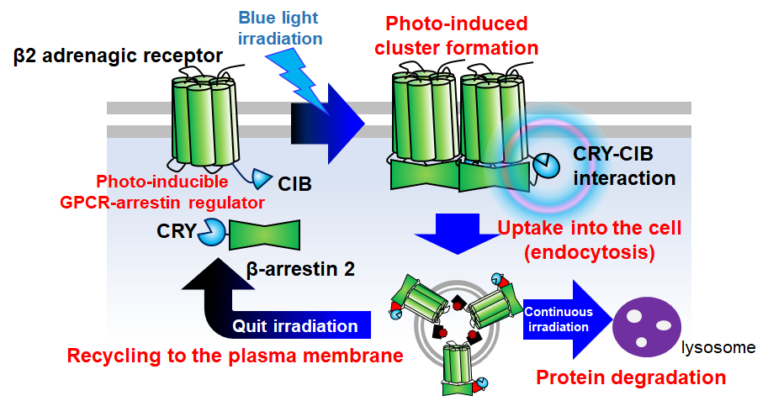
This principle of optical manipulation of plasma membrane receptors can be applied to a variety of receptors and is expected to contribute to the elucidation of the biological roles of dynamic intercellular signaling mediated by receptors in vivo.
- M. Endo, T. Iwawaki, H. Yoshimura, T. Ozawa, ACS Chemical Biology. 14, 2206–2214 (2019).
- O. Takenouchi, H. Yoshimura, T. Ozawa, Scientific Reports. 8, 677 (2018).
- M. Endo, M. Hattori, H. Toriyabe, H. Ohno, H. Kamiguchi, Y. Iino, T. Ozawa, Scientific Reports. 6, 23976 (2016).
