Proteases, peptide-cleaving enzymes, are used for signal transduction such as cell death (apoptosis), cancer cell invasion, and human immunodeficiency virus proliferation These signals play an important role in regulating cellular functions, and protease-targeted drugs are currently being developed. Although development of fluorescent probes to detect the protease activity has been vigorously pursued worldwide, it is not easy to observe the activity in the deep tissues in animals. We have developed a probe that can detect apoptosis in deep tissues by utilizing luciferase (Luc).
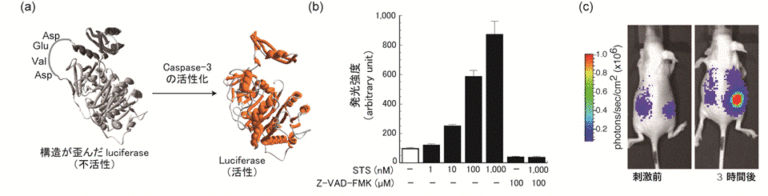
The probe consists of a luciferase with a cyclic structure, in which the N- and C-termini are linked by peptide bonds. The cyclic Luc loses its activity due to distortion of its conformation. This inactive cyclic Luc is expressed in cells and stored intracellularly. If a protease is activated in the cell, the cyclic Luc is cleaved and returns to its original conformation, and luminescence can be detected. By expressing Luc in animal livers, we have demonstrated that hepatocyte apoptosis can be detected noninvasively (1-3). The molecular design of the cyclic Luc has also been applied to the detection of the cleavage activity of the cell membrane protein CD44 (4), and it has general applicability to evaluate the target protease activity by changing the sequence to a protease recognition sequence.
- Kanno, Y. Yamanaka, H. Hirano, Y. Umezawa and T. Ozawa, Angew. Chem. Int. Ed. 46, 7595-7599 (2007).
- M. Ozaki, S. Haga and T. Ozawa, Theranostics, 2, 207-214 (2012).
- S. Haga, T. Ozawa, Y. Yamada, N. Morita, I. Nagashima, H. Inoue, Y. Inaba, N. Noda, R. Abe, K. Umezawa, M. Ozaki, Antioxid. Redox Signal., 21, 2515-2530 (2014).
- N. Noda and T. Ozawa, J. Cell Sci., 135, jcs259314 (2022).
